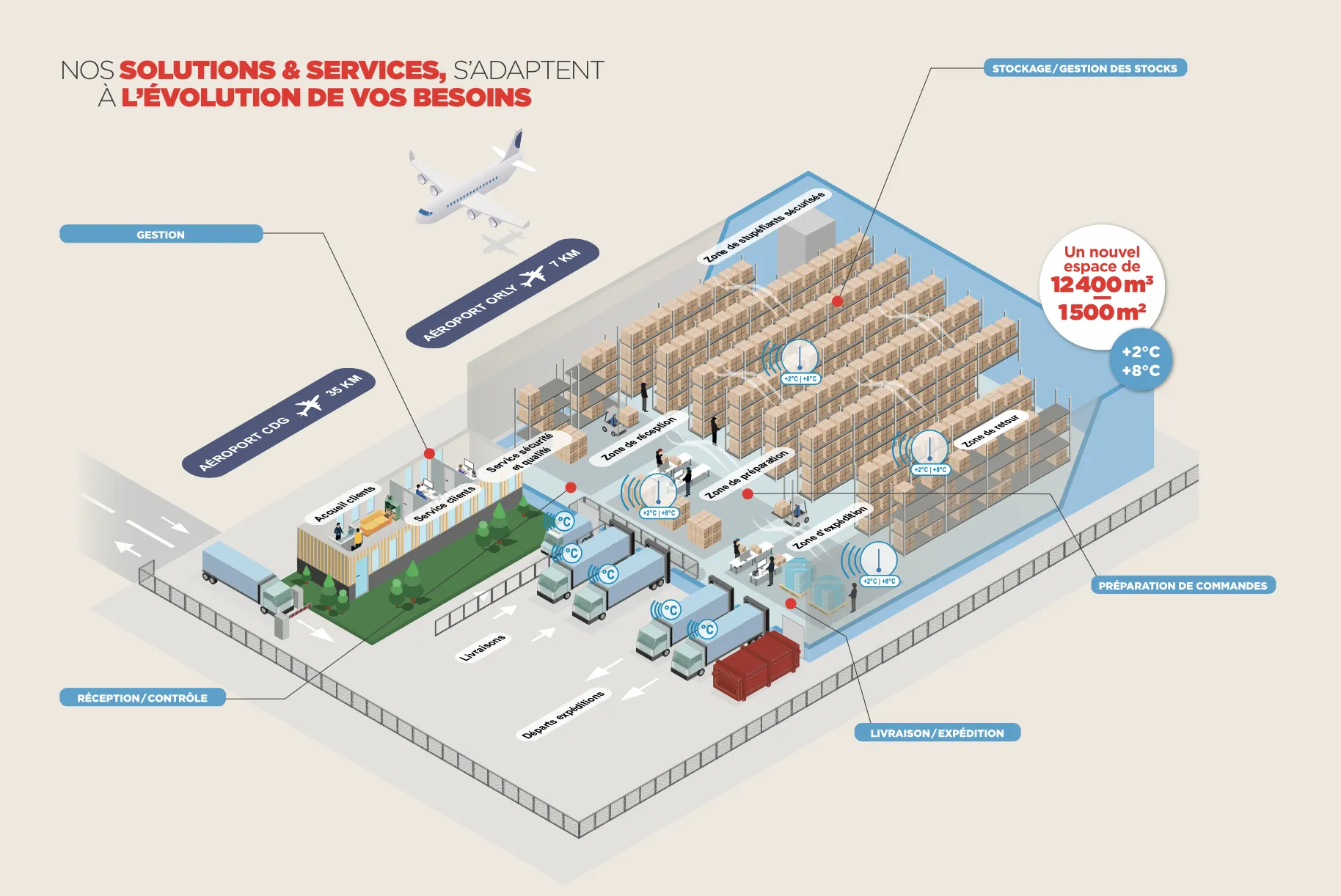
TEDIS has created a new storage and service solution dedicated to the pharmaceutical cold chain.
Meeting your expectations is at the core of our commitment.
Our teams of experienced, available, and specialized employees support you at every step of the process. We analyze your needs and formulate a tailored logistics solution in terms of responsiveness, costs, and quality.

TEDIS has established a new space of 12,400 m³/1,500 m² (+2°C/+8°C) in the Paris region which allows us to meet all the storage and distribution needs for temperature-sensitive medications, vaccines, and laboratory reagents.
We offer our partners the opportunity to expand their storage space sustainably or temporarily, have additional remote stock, accommodate an increase in orders, and benefit from a transition hub or storage towards other export territories while ensuring the permanent availability of their products.
We design and develop a range of “customized services” that adhere to all international standards.